Day 1 :
Keynote Forum
Ioannis V Yentekakis
Technical University of Crete, Greece
Keynote: Ionically conducting materials as effective catalyst supports with potential implementations in emissions control catalysis
Time : 10:15-10:40

Biography:
Ioannis V Yentekakis is Full Professor of Physical Chemistry and Catalysis in the School of Environmental Engineering, Technical University of Crete (TUC), Greece. He received Chemical Engineering Diploma (1983) and PhD (1988) from the University of Patras. He has joined Princeton University USA, ICE-HT/FORTH Patras GR, Cambridge University UK as Senior Researcher, University of Patras, Dept. Chemical Engineering, as Assistant Professor (1996-2000), and finally Technical University of Crete as Associate (2001-06) and Full Professor (2006-today). He is regular member of the University Council of Technical University of Crete and Director of the Laboratory of Physical Chemistry & Chemical Processes. His current interest concerns development of novel materials and processes in heterogeneous catalysis for green and fine chemistry, environmental protection and renewable/sustainable energy generation. He authorized >100 peer-reviewed journal publications (with >3000 citations, h-index=32), >120 conference proceedings, 3 patents and 10 books
Abstract:
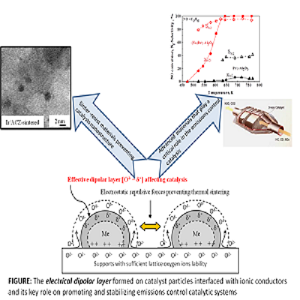
Statement of the Problem: Nowadays, the abatement of CO, HCs, NOx and N2O emissions from automotive or stationary sources constitutes a subject of major environmental importance because of the major contribution of these pollutants to serious environmental problems, such as photochemical smog, acid rain, greenhouse effect and climate change as well as stratospheric ozone depletion (N2O). Heterogeneous catalysis plays a key role in pollutant abatement technologies and often provides the most attractive and efficient solutions as, for example, in automotive emissions control – the most significant source of atmospheric pollution over the word. However, atmospheric pollution remains a huge and growing problem; therefore, an imperative need of even more efficient and economic catalytic abatement technologies remains as a highly desirable goal. An advanced catalyst promotion method that provides catalytic systems with exceptional activity and stability has currently attracted extensive attention for a wide range of applications related to energy production and environmental protection, is the subject herein.
Methodology & Theoretical Orientation: Ionically conducting materials as catalyst supports can be used as tunable metal-support interaction carriers, effectively controlling catalytic properties, via an effective dipolar layer of ionic promoter species formed at the catalyst particle surfaces, with concomitant dramatic effects on catalytic performance. The dipolar layer and its intensity (promoter species population), can be electrochemically controlled (Electrochemical Promotion or NEMCA effect) or can spontaneously be created on traditional-type highly dispersed catalysts, via thermally-driven spillover of ionic species from the support on the nanoparticle surfaces.
Conclusion & Significance: Worth noting achievements on environmentally important catalytic reactions (CO, HCs, NOx, N2O abatement) have been accomplished by this concept of promotion. An additional implementation of the concept, which concerns catalyst nanoparticles stabilization against thermal sintering, a subject of great importance in industrial heterogeneous catalytic processes, has recently been discovered.
- Environmental Chemistry | Environmental Toxicology and Mutagenicity| Renewable Energy Sources and Storages
Location: Olimpica 2, Holiday Inn Rome Aurelia
Session Introduction
Haider A Khwaja
University at Albany, USA
Title: An assessment of air quality in the surrounding holy places of Mecca, Saudi Arabia during Hajj
Time : 14:00-14:20

Biography:
Dr. Haider A. Khwaja has twenty-seven years of solid research experience on environmental projects. He is the Director of the Laboratory of Inorganic Chemistry at the Wadsworth Center, NYSDOH. Dr. Khwaja is a faculty member at the Department of Environmental Health Sciences, School of Public Health, University at Albany, where he teaches and mentors graduate and undergraduate students. He has been carrying out various environmental projects in USA and in developing countries. Active research programs include:
• Effects of particulate matter on daily morbidity and mortality due to cardiovascular and pulmonary diseases in urban areas
• Chemical characteristics of fine particles responsible for the observed health effects
• Exposure and health impacts related to outdoor and indoor air pollution including studies of volatile organic compounds (VOCs), polycyclic aromatic hydrocarbons (PAHs), diesel vehicle emission, air toxins, and indoor allergens
Water and human health issues such as drinking water quality and human health risks associated with water contaminants.
Abstract:
The associations of exposure to air pollution and adverse human health effects have been demonstrated in many epidemiologic studies. Hajj, an annual pilgrimage of Islam, draws millions of pilgrims from more than 200 countries for religious rituals in Mecca, Saudi Arabia. The city is surrounded by mountains with a population of 1.7 million, which gets doubles or even more during Hajj. The city centers on the Grand Mosque (Masjid Al-haram), connected with the network of tunnels. Main Hajj pilgrimage route for five days extends 20 km to the east and includes “Mina”, “Arafat”, and “Muzdalifah”. A detailed study was conducted in Mecca, its tunnels, and surrounding holy places during Hajj (October 13-17, 2013). Spatial and temporal variations in total suspended particulate (TSP), PM10 , PM7 , PM2.5 , PM1 , ozone (O3), and black carbon (BC) levels along the route were recorded using portable monitors and GPS to assess the status of air quality. This is the first study to elucidate the exposure to air pollutants among pilgrims. Extremely high levels of all pollutants were observed during the intensive measuring periods. For example, the PM7, PM2.5, O3, and BC concentrations of up to 9,433 µg/m3, 484 µg/m3, 444 ppb, and 468 µg/m3, respectively, were observed. Results of this investigation revealed that most routes had on average exceeded the World Health Organization (WHO) standards for PM10 and PM2.5. The reasons for the high air pollutants concentrations are most probably high volume of traffic, construction work, re-suspension of particles, and geographical conditions (arid regions). The pilgrim’s longer trip duration lead to their highest whole trip exposure to air pollutants, which indicate that they are possibly subject to higher health risk. Better understanding of air pollution exposure and their determinants in the environments will contribute to the development of more appropriate exposure reductive strategies and have significant public health meanings.
Angelo Nacci
University of Bari, Italy
Title: Nanostructured materials for green catalysis
Time : 14:20-14:40

Biography:
Angelo Nacci completed his PhD in Chemical Sciences in 1994 at Bari University (Italy). He was a Researcher in Organic Chemistry at the Chemistry department of Bari University. In 2001, he was a Visiting Researcher at TUM University of Munich (Germany) and in 2005 he became an Associate Professor of Organic Chemistry. He is currently the President of Chemistry Courses Degree at Bari University. His research interests are focused on: organometallic chemistry in ionic liquids; green nanocatalysis; CO2 capture and valorization and synthesis and recycling of bioplastics. He is the co-author of almost 70 publications in major journals and 1 patent.
Abstract:
Transition-metal nanoparticles (NPs) are attracting a great deal of attention in almost any scientific and technological field, including catalysis, where nanoscale materials are becoming more prevalent in a wide range of applications such as fuel conversion, pollution abatement and fine chemical production. Nowadays, many researchers are exploiting the high activity and selectivity of nanocatalysts to develop greener and waste-minimized processes. During the last decade, we exploited nanostructured catalysts based on several metals like Pd, Cu, Au, Zn and Ti to perform a wide range of organometallic reactions (Heck, Suzuki, Ullmann, Stille, carbonylations, cyclopropanations, C-H activations, hydrodehalogenations and CO2 photoreduction) under environmentally friendly conditions given by the absence of phosphane ligands and using neoteric solvents (ionic liquids, water, emulsion mixtures and so on) as green reaction media. This lecture deals with our recent advances in controlling the catalyst performances by choosing properly the nature of both the ionic liquid and the nanocatalyst.
Dominik Rohrmus
Siemens AG, Germany
Title: Carbon-enhanced manufacturing and digitalization supporting cycle economy
Time : 14:40-15:00

Biography:
Dominik Rohrmus works at Siemens Corporate Technology in Munich, Germany in different functions in the area of manufacturing development and production equipment realization since 2005. In 2009, he founded the company program, Sustainable Production Engineering and rolled several demonstrator projects company-wide out. In particular energy efficient production planning and technology on the shop-floor set the focus of that program. Also cycle economy and cycle business development in cooperation with Siemens business units and external partners is part of the program and yield already several pilot projects. Since 2013, he is the Head of the research group, Manufacturing Systems Engineering at Siemens Corporate Technology. The research group is responsible for shop-floor equipment standardization and development of the future for the Siemens factories worldwide.
Abstract:
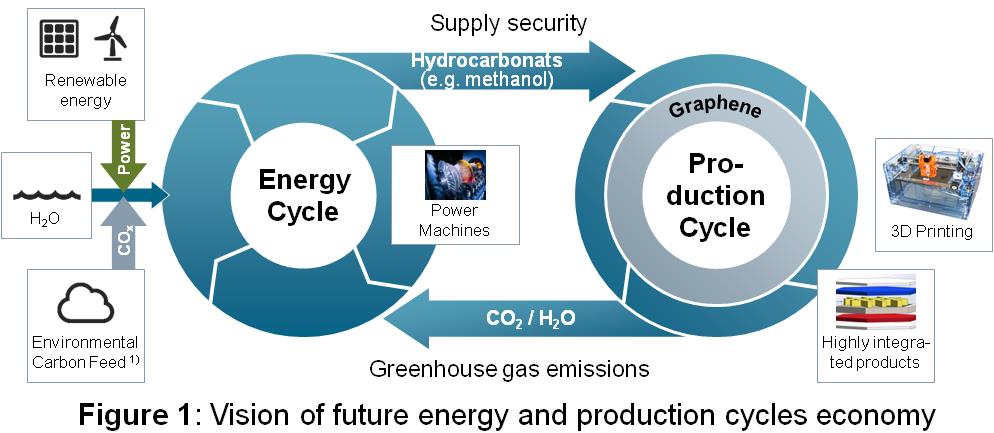
The global challenge climate change calls for answers beyond the pure optimization of resources and energy consumption in manufacturing - a major CO2 causer. CO2 as a supply for new synthetic raw materials and products as well as markets is a new long-term approach to establish a green cycle economy. We define green cycles as CO2 sinks. The Siemens green cycle vision for green production and green raw materials as displayed in Figure 1 opens new manufacturing models and new product markets to provide an answer for the world’s hunger for materials. These materials have a promising future for non-food related components such as electronic parts. Green cycle factories apply the concept of green cycles to the discrete manufacturing industries. The prerequisites are renewable energy and chemistry production technologies, which are synthesizing fuels and materials for manufacturing from CO2 sources. The world has to focus on renewable supplies, which fulfill the demands of future manufacturing technologies in terms of additive manufacturing processes that are then mainly using carbon materials. Our new findings can help to contribute to a greener future as carbon-based materials come from renewable, biodegradable resources. The transformation process requires new competitive manufacturing systems in a decentralized and digitalized manner. An additive manufacturing process based on this carbon feedstock is one promising application field with the advantage to transfer the carbon load into discrete products. Hence, low carbon in the atmosphere can be realized by green cycles and advanced carbon-based materials and manufacturing.
Maria Michela Dell’Anna
Polythecnic University of Bari, Italy
Title: Catalytic activity and recyclability of polymer supported palladium or nickel nanoparticles in organic reactions in water
Time : 15:00-15:20

Biography:
Dr Dell’Anna completed her PhD in “Chemistry of materials for special uses” at University of Reggio Calabria (Italy), giving a dissertation on “Synthesis and characterization of new transition metal complexes. Aerobic oxidation of organic substrates and C-C bond forming reactions”.
During her PhD studies, M.M. Dell'Anna joined for one year the research group of Prof. M. Cowie in the Chemistry Department of University of Alberta (Canada), where she worked on the synthesis and characterization of bimetallic complexes.
Since 2000 she has been Assistant Professor in Chemistry at Polytechnic of Bari.
Since 2012 she has been the Editor of the journal “Recyclable Catalysis”, merged with the journal “Catalysis for Sustainable Energy”.
Research interests are focused on: i) polymer supported metal catalysts; ii) green nanocatalysis; iii) platinum complexes and iv) risk assessment. She is co-author of almost 45 publications on major journals and more than 20 Communications to Congress.
Abstract:
An insoluble palladium catalyst (Pd-pol) was obtained by copolymerization of the metal containing monomer Pd(AAEMA)2 [AAEMA− = deprotonated form of 2-(acetoacetoxy)ethyl methacrylate] with ethyl methacrylate (co-monomer) and ethylene glycol dimethacrylate (cross-linker), followed by in situ reduction of Pd(II) to Pd(0), to give polymer stabilized metal nanoparticles. The good swellability in water exhibited by Pd-pol rendered it an ideal potential catalyst for reactions carried out in a green solvent, such as water, since the migration of the reagents to the active sites would not be hampered by the solid support. With the aim to develop innovative catalytic processes that enable chemical transformations to be performed under mild and sustainable conditions with high efficiency, we decided to evaluate the catalytic activity of Pd-pol for several important organic reactions using water as solvent. Pd-pol resulted highly active and selective in catalyzing (figure 1): the Suzuki-Miyaura coupling between aryl bromides or activated aryl chlorides and phenylboronic acid; the oxidation of benzyl alcohols to aldehydes; the reduction of quinolines and nitroarenes by H2 or NaBH4. Pd-pol was recyclable for several consecutive runs (for example, at least 12 times in the nitroarene reduction). TEM analyses carried out on the catalyst showed that the active species were supported palladium nanoparticles having a mean size of 4 nm, which did not aggregate with the recycles. Recently, due to their low cost, Ni catalysts have been employed in several organic reactions (mainly hydrogenations). In this context, we synthetized a Ni catalyst similar to Pd-pol, starting from Ni(AAEMA)2 and we employed it as active and recyclable, insoluble catalyst for the reduction of different nitroarenes to give the corresponding anilines, under sustainable conditions. All these results proved that the proposed Pd or Ni based composite materials are excellent hybrid structures as efficient and reusable catalysts.
Sijun Dong
Institute of Urban Environment - CAS, China
Title: A novel mechanism for BPA-triggered hepatic steatosis
Time : 15:20-15:40

Biography:
Sijun Dong has completed his PhD at 2004 in Biochemistry and Biotechnology, Tokyo University of Agriculture and Technology, Japan. He is the professor of Environmental science, Chinese Academy of Sciences. He has published more than 50 papers in reputed journals. His research interest is about Environmental Molecular Toxicology and Chinese Medicines and Environmental Health.
Abstract:
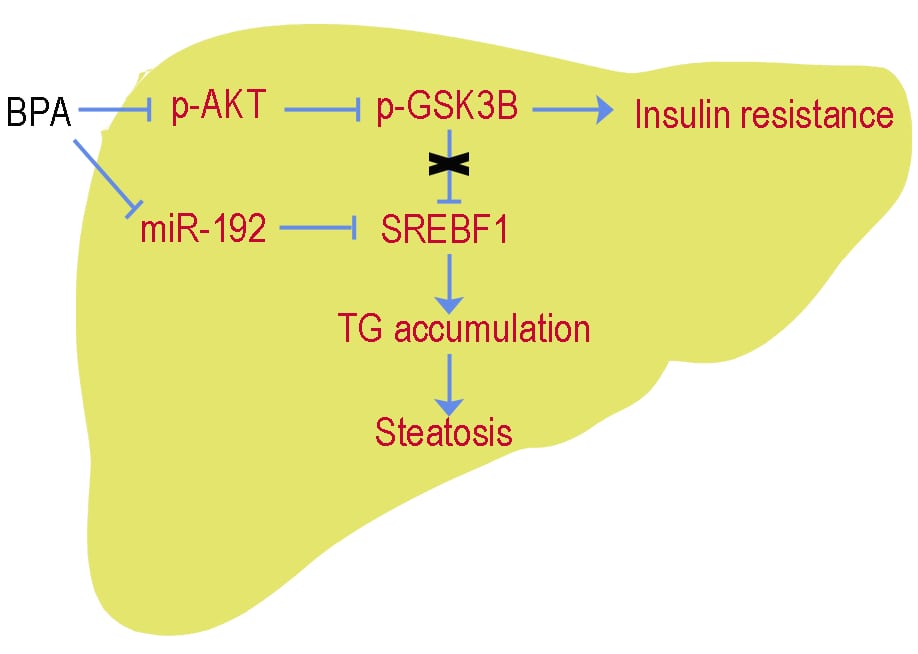
Exposure to bisphenol A (BPA) has been linked to the increased incidence of non-alcoholic fatty liver disease (NAFLD): the hepatic manifestation of metabolic syndrome. However, the related underlying mechanisms remain unknown. Given that microRNAs (miRNAs) are widely recognized as the key regulators of lipid metabolism and the potential mediators of environmental effects, this study aimed to examine whether exposure to BPA triggered hepatic lipid accumulation and to further determine if adverse effects of BPA may be modulated in part through miRNAs. Male post-weaning C57BL/6 mice were exposed to 50 μg/kg/day BPA or corn oil for 90 days by oral gavage. We found that insulin resistance, and impaired hepatic lipid accumulation and increased serum triglycerides (TG) existed concomitantly in the BPA exposed mice. In addition, BPA exposure caused significant reduction in miR-192 expression in both mice liver tissues and human HepG2 cells, which were accompanied by significant up-regulation of SREBF1 (a key transcription factors that is capable of activate lipid synthesis) and subsequent expression of lipogenic genes. Bioinformatic and in vitro studies suggested that miR-192 acted to the 3’UTR of SREBF1 directly, resulting in profound dysregulation in hepatic lipid homeostasis. Inhibition of miR-192 led to higher TG levels and increased hepatic lipid accumulation by enhancing SREBF1 processing. In contrast, the opposite results were observed with overexpression of miR-192, which downregulated SREBF1 expression. Most importantly, we also showed that in vivo and in vitro overexpression of miR-192 effectively prevented BPA induced hepatic lipid accumulation, which was independently of insulin resistance. In conclusion, this study showed a novel mechanism that exposure to BPA may up-regulate SREBF1 through inhibition of miR-192 in the liver, thereby contributing to NAFLD.
Khalaf M Alenezi
University of Hail, KSA
Title: Electrocatalytic production of hydrogen using iron sulfur cluster
Time : 15:40-16:00

Biography:
Abstract:
In response to the energy crisis, rising fossil fuel costs and global climate warming, this study focuses on the electrocatalytic reduction of proton into hydrogen using an iron sulfur cluster in the presence of pentafluorothiophenol. The direct reduction of pentafluorothiophenol at vitreous carbon electrode occurs at Ep -1.3 V vs. Ag/AgCl in [Bu4N][BF4]-DMF solution. Interestingly, in the presence of [Fe4S4(SPh)4][Bu4N]2, the reduction potential shifts significantly to -0.98 V vs. Ag/AgCl. Based on gas chromatography analysis, the formation of H2 has been confirmed with a current efficiency of catalyst. 63% after two hours, while the chemical yield at the carbon electrode was about 46%. On the other hand, no H2 gas was detected without catalyst. Importantly, the increment of the concentration of acid (up to 18 equivalents) led to a positive shifting in the reduction potential until a value of 0.18 V. These results reflect the exquisite electrocatalytic efficiency of the protein-like iron sulfur cluster in Hydrogen Evolution Reaction (HER).
Gökberk Kara
Marmara University, Turkey
Title: Heavy metals in seawater from Marmara sea, Istanbul
Time : 16:15-16:35

Biography:
Gokberk Kara has completed his undergraduation in the Environmental Engineering department of Sakarya University in 2007. He is a Master’s degree student in Environmental Engineering department of Marmara University and is also working at the RSK Environmental Consultancy Service in Istanbul, Turkey.
Abstract:
In Istanbul, where dense industrialization and dense population are located, people can use Marmara Sea for recreational reasons. The beaches in which they interacted with the sea were selected primarily for the determination of heavy metal pollution. This study proposed to investigate the quality of seawater to protect health of people which do recreational activities such as swimming. Cr, and Zn concentrations and pH, temperature in seawater samples taken weekly from Kucuksu, Fenerbahce and Suadiye beaches in Asian side of Istanbul were investigated between 9 February 2009 and 4 May 2009. Measured temperature values varied from 7.1 to 27.60C and measured pH values varied from 8.15 to 8.47. The Cr and Zn concentrations analyzed by the Inductively Coupled Plasma Mass Spectrometry (ICP-MS) were under the limit values by the Turkish Water Pollution Control Regulation published by the Ministry of Environment and Urbanization. These findings indicate that there is no significant threat to aquatic life and human health from these heavy metals in sea water in Marmara Sea.
Acknowledgments
The directorate of Scientific Research Projects (BAP) of Marmara University, grant number FEN-D-120813-0355 supported this project.
Jayalal L P R Wijesinghe
National Water Supply and Drainage Board, Sri Lanka
Title: Dosing of proper oxidizing agents pretreatment to Kalatuwawa water to reduce the Trihalomethane (THM) formation in drinking water
Time : 16:35-16:55

Biography:
Jayalal L P R Wijesinghe graduated from University of Colombo, Sri Lanka with a BSc (Hons) degree. After graduation he has joined the Department of Chemistry, University of Colombo as a Demonstrator then as a Researcher. He has obtained PG Diploma in the field of Toxicology and MSc degree in the field of Analytical Chemistry from the same university. He has started his water sector career as a Regional Chemist attached to the National Water Supply & Drainage Board in North Central Province of Sri Lanka. After successful completion of eight years of regional works, he was promoted to the Senior Chemist position in NWSDB. Presently he is working as a Chief Chemist in NWS&DB in Sri Lanka. He has gained 22 years of experience in the field of Water Quality Monitoring with the operation of high end analytical instruments – AAS with GTA, GC/MS, etc. He has published twelve research papers in local and international journals. He has undergone many training programs locally as well as internationally – Lake Water Quality Management course in Japan, LC/MS training course at Waters in India, Analytical Skills Development Course in Helsinky University in Finland, etc. He is a Fellow Member of the Institute of Chemistry, Ceylon and also a Chartered Chemist.
Abstract:
Kalatuwawa water treatment plant consists of aeration, coagulation, flocculation, sedimentation, filtration and disinfection. Existing aeration is not enough to oxidize the inorganic and organic impurities in Kalatuwawa raw water, therefore prechlorine is used as oxidizing agent in Kalatuwawa. Analytical results revealed that during the years 2000–2012, the Kalatuwawa raw water contained high concentration of ammonia (0.578±0.469 mg/dm3), and highest concentration of ammonia 1.92 mg/dm3 was reported at the bottom of the reservoir, high concentration of iron (0.751±0.643mg/dm3), and the highest concentration of iron 12 mg/dm3 was reported at the bottom of the reservoir. The color levels were 11.571±7.271 Hz and the highest level of color, 90 Hz was reported at the bottom of the reservoir. Pre chlorination where chlorine is utilized not only oxidize the iron, manganese, ammonia and organic compounds but also reacts with naturally occurring organic matter present in water to produce a group of organic compounds as a byproduct arising from chlorination which was classified as trihalomethane (THM). THMs are often used as indicator compounds for other DBPs. The four main THM compounds found in abundance are chloroform (CHCl3), bromodichloromethane (CHCl2Br), dibromochloromethane (CHClBr2) and bromoform (CHBr3). Many factors which will affect the THM formation are concentration and type of precursors, type of disinfectant and concentration and the dosing point, temperature, pH, contact time and the length of the distribution network. This study is basically investigating THM formation without prechlorination where potassium permanganate (KMnO4) is used as pretreatment to remove the organic and inorganic impurities. Laboratory analysis was carried out using Jar test apparatus with potassium permanganate to remove the impurities. Analytical results revealed that 90% of iron was removed with 0.3-0.6 mgdm-3 dosage of potassium permanganate and the same time manganese level is within SLS 614: 2013. THM levels were monitored using GC–ECD couple to purge and trap system where the THM levels were reduced significantly (67%).
Linping Zhang
Donghua University, China
Title: Preparation of Yb3+ doped microspherical BiOI and its photocatalytic activity for the degradation of rhodamine B in water
Time : 16:55-17:15

Biography:
Linping Zhang has completed her PhD at the age of 30 years from The Chinese University of Hong Kong. She is an associate professor of Donghua University in China. She has published more than 45 papers in reputed journals.
Abstract:
Bismuth oxychloride (BiOI) has a good visible light responsive property due to their relatively narrow band gap, and its photocatalytic performance is further improved by doping Ytterbium ions (Yb3+) which could be attributed to stronger optical absorption in UV-visible light region, effective separation of the photogenerated electron-hole pairs, and the capacity of Yb3+ ions to up-convert Near-IR light into visible-light and UV light. In this study, a facile solvothermal method was adopted to synthesize different Yb3+ ions doped BiOI photocatalysts. The doped photocatalysts with molar ratios of 0, 0.5, 1, 1.5, 2 and 2.5% Yb3+ ions were prepared and 2% Yb3+ ions doped BiOI exhibited the highest photocatalytic degradation efficiency, which was 2 times higher than that of pure BiOI. As-prepared photocatalysts were further studied through SEM, XRD, UV–vis DRS and free radical capture experiments, etc. which indicated that the doping ions entered into Lattice of BiOI photocatalysts and improved the photocatalytic performance. This work provided some potential application of Yb3+ doped BiOI for the degradation of organic contaminants in water.
Adebayo Oluwafemi Lawrence
College of Education Ikere, Nigeria
Title: Concentration, temperature and kinetics studies of Kola nitida leaves extract in corrosion prevention
Time : 17:15-17:35

Biography:
Adebayo Oluwafemi Lawrence has completed his Master’s degree in Chemistry from Adekunle Ajasin University, Nigeria in the year 2014. He is currently pursuing his PhD in Chemistry at the Federal University of Technology, Nigeria. He is currently working as a Lecturer in the Department of Chemistry, College of Education Ikere, Nigeria. He has published 10 papers in reputed journals.
Abstract:
Weight loss test method was used to study the inhibition and adsorption properties of Kola nitida leaves extract addition on corrosion inhibition efficiency of mild steel in 2 M HCl solution for various concentration, exposure time and working temperature. The results showed that ethanolic extract of Kola nitida leaves is a potential inhibitor for the corrosion of mild steel in acidic medium. The corrosion rate of mild steel in 2 M HCl decreases with increasing in the concentration of the extract. The inhibition efficiency increases progressively as the concentration of the extract increases but decreases with rise in temperature and exposure time. The highest inhibition efficiency observed in the presence of the extract was 88.51%. The kinetics of the reaction in the presence of the extracts revealed that it follows a first order reaction and the half-life increases as the concentration of the extract increases. Adsorption studies revealed that Langmuir adsorption isotherm is the best adsorption model applicable to the adsorption of the extract on mild steel surface.
Ubwa S T
Benue State University, Nigeria
Title: Assessment of Polynuclear Aromatic Hydrocarbons (PAHs) in local steak (suya) samples in Makurdi town, Benue State-Nigeria
Time : 17:35-17:55

Biography:
Ubwa S T has his expertise in Food and Environmental Organic Chemistry. He has pioneered the analysis of common environmental food contaminants at the Benue State University Makurdi. He was one of the team members that attracted the World Bank grant to the University’s center of excellence in food technology and research. His work focuses in these areas: the analysis for total aflatoxins, and polynuclear aromatic hydrocarbons in smoked and stored foods in Benue Sate-Nigeria.
Abstract:
Statement of the Problem: Owing to the methods applied in the preparation of local steak (Suya) in Nigeria, polycyclic aromatic hydrocarbons (PAHs) are likely to be formed in it; as their formation result from the incomplete combustion. Taking the quality and safety of Suya for human consumption into consideration, it becomes imperative to determine polynuclear aromatic hydrocarbons (PAHs) in Suya samples.
Methodology & Theoretical Orientation: This study determined the presence of polynuclear aromatic hydrocarbons (PAHs) in Suya samples collected from 20 different locations in South and North Banks of Makurdi, Benue State. With the use of ultrasonic agitation, the samples were extracted, and were further fractionated and subjected to GC-FID instrumentation afterward.
Findings & Conclusion: The results obtained from the analysis shows that individual PAHs were present in very low concentrations but the concentration of combined PAHs was generally high in each of the 20 samples. The levels of total PAHs (0.017 to 0.069 mg/kg) found in all the samples were higher than the recommended maximum residual limits (MRLs) for benzo[a]pyrene of 5 μg/kg (0.005 mg/kg) set by the commission of European Communities (EC) for smoked meat and smoked meat products. All the samples had relatively higher levels of high molecular weight (HMW) PAHs than low molecular weight (LMW) PAHs. The high content of total PAHs (especially HMW PAHs) through bioaccumulation may be detrimental to human health and thus alternative methods of Suya preparation are recommended.
Miada A Ali
United Arab Emirates University, UAE
Title: Using electrocoagulation to remove chloride and ammonium from reject brine treated by solvay process
Time : 17:55-18:15

Biography:
Miada A. Ali has completed her bachelor at the age of 26 years from UAE University. Since 2014, she joined United Arab Emirates University as a research assistant. Currently, she is a post graduate student in Chemical Engineering Department at the United Arab Emirates University.
Abstract:
The excessive use of desalination, which is due to the increase in fresh water demand, results in large productions of reject brine. Therefore, the development of an efficient treatment process of the reject brine becomes vital. The Solvay process is one of the main treatment technologies, wherein NH3 is introduced to converting soluble Na+ into insoluble NaHCO3. Although the concentration of Na+ ions are reduced in this process, Cl- ions are not affected. In addition, the concentration of ions increase. Electrocoagulation has been tested for the remove Cl- and ions and to regenerate the NH3. When no current was applied, a very small removal of the ions was recorded. Applying a current density of 0.1167 A/cm2 caused an increase in the removal of and Cl-, by 45.9% and 25.87%, respectively at 20°C, 71.55% and 26.88%, respectively at 30°C. This clearly proves that the main removal was due to the electrocoagulation. The effects of current density, initial concentration, and temperature have been studied on the ions removal. It was found that increasing the current density and/or the temperature was found to decrease the residual ions concentration. Whereas, increasing the initial ions concentration resulted in decreasing the percentage removal.
- Environmental Toxicology and Mutagenicity




